Background: In CLL/SLL, ibrutinib treatment before leukapheresis improved in vivo and ex vivo expansion of the CD19-directed chimeric antigen receptor (CAR) T cell therapy tisagenlecleucel, and concurrent ibrutinib therapy improved engraftment and therapeutic efficacy of anti-CD19 CAR T cells in human xenograft mouse models (Fraietta et al. Blood. 2016;127:1117-27). Recent studies in patients with R/R CLL suggest that CD19-directed CAR T cell therapy combined with ibrutinib improves response rates with CTL119 and JCAR014 (Gill et al. Blood. 2018;132:298; Gauthier et al. Blood. 2020;135:1650-60). Liso-cel is an investigational, CD19-directed, defined composition, 4-1BB CAR T cell product administered at equal doses of CD8+ and CD4+ CAR+ T cells. We report initial safety and preliminary efficacy from the phase 1 liso-cel and ibrutinib combination cohort of the ongoing phase 1/2 TRANSCEND CLL 004 study (NCT03331198) in patients with R/R CLL/SLL.
Methods: Eligible patients with CLL/SLL met ≥1 of the following: 1) received ibrutinib and progressed at time of study enrollment; 2) had high-risk features and received ibrutinib for ≥6 months (mo) with less than a complete response (CR); 3) had a Bruton tyrosine kinase (BTK) or PLCγ2 gene mutation, with or without progression on ibrutinib; 4) had received prior ibrutinib with no contraindication to reinitiating ibrutinib. Baseline disease assessments included bone marrow (BM) biopsy, complete blood count, lymphocyte enumeration, and CT scan. At enrollment, patients started or continued ibrutinib. Patients continued ibrutinib through leukapheresis and for ≥90 days after liso-cel infusion. Patients received liso-cel infusion at 50 × 106 (dose level [DL]1) or 100 × 106 (DL2) CAR+ T cells after 3 days of lymphodepletion with fludarabine/cyclophosphamide. Primary endpoints were safety and to determine the recommended dose (RD) of liso-cel in combination with ibrutinib for R/R CLL/SLL; overall response (OR) rate (CR + CR with incomplete blood count recovery [CRi] + partial response) and pharmacokinetics (PK) were exploratory endpoints. The RD was selected based on the modified toxicity probability interval algorithm.
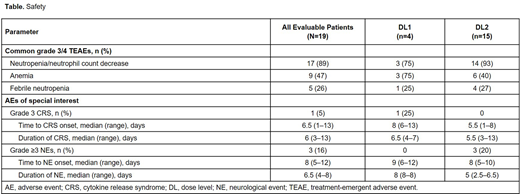
Results: At data cutoff, 19 patients received liso-cel (DL1, n=4; DL2, n=15) with ibrutinib. Median age was 60 (range, 50‒77) years, and 18 patients (95%) had high-risk cytogenetics (del[17p], n=8; TP53 mutation, n=6; unmutated IGHV, n=16). Patients had a median of 4 (range, 2‒11) prior therapies. All patients were R/R to prior ibrutinib; 14 patients (74%) had BTK inhibitor as last prior therapy and 10 (53%) had prior venetoclax. No dose-limiting toxicities were observed at either DL. The most common grade ≥3 treatment-emergent adverse events (TEAEs) were neutropenia/neutrophil count decrease (n=17; 89%), anemia (n=9; 47%), and febrile neutropenia (n=5; 26%; Table). Six patients had infections at DL2: grade 3 and grade 2 lung infection (n=1 each) and grade 2 coccidioidomycosis, scabies, skin, and gum infections (n=1 each). Ibrutinib-related AEs included diarrhea (n=7), hypertension (n=4), atrial fibrillation (n=1), and rash (n=1). No grade 5 TEAEs occurred. Fourteen patients (74%) had cytokine release syndrome (CRS; 1 grade 3) and 6 (32%) had neurological events (NEs; 3 grade ≥3). Seven patients (37%) required tocilizumab and/or corticosteroids to manage CRS and/or NEs. Preliminary PK data showed a median time to peak liso-cel expansion of 11 days across DLs (DL1, 12 days; DL2, 11 days). Of 19 patients with ≥1-mo follow-up, 18 (95%) had an OR (DL2, 100%; DL1, 75%) and 9 (47%) had a CR/CRi. One patient (5%) had stable disease. All ORs were achieved by Day 30 postinfusion, and 15 (83%) of 18 patients maintained their response at 3-mo follow-up. Of 19 patients evaluable for minimal residual disease (MRD), 17 (89%) achieved undetectable MRD in blood via flow cytometry and 15 (79%) in BM by next-generation sequencing (both sensitivity of ≤10-4).
Conclusions: Preliminary data show that liso-cel in combination with ibrutinib is associated with manageable safety, including a low incidence of grade 3 CRS and grade ≥3 NEs, and promising efficacy in heavily pretreated patients with R/R CLL/SLL. No clear difference in safety was observed across DLs, and DL2 was selected as the RD for liso-cel in combination with ibrutinib in patients with R/R CLL/SLL. Updated results from the full combination cohort and additional PK/pharmacodynamic data will be reported.
Dorritie:Juno Therapeutics: Research Funding; Kite-Gilead: Research Funding. Munoz:Portola: Research Funding; Incyte: Research Funding; Acrotech/Aurobindo: Speakers Bureau; Alexion: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Fosunkite: Consultancy; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Innovent: Consultancy; Genentech/Roche: Research Funding, Speakers Bureau; Pfizer: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Juno/Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Millenium: Research Funding; Verastem: Speakers Bureau; Merck: Research Funding; AbbVie: Consultancy, Speakers Bureau; AstraZeneca: Speakers Bureau; Kyowa: Consultancy, Honoraria, Speakers Bureau. Stephens:Innate: Consultancy; Verastem: Research Funding; Beigene: Consultancy; Karyopharm: Consultancy, Research Funding; Acerta: Research Funding; Gilead: Research Funding; Juno: Research Funding; MingSight: Research Funding; Arqule: Research Funding; Janssen: Consultancy; Pharmacyclics: Consultancy. Gillenwater:Juno Therapeutics, a Bristol-Myers Squibb Company: Current Employment; Bristol-Myers Squibb: Current equity holder in publicly-traded company. Gong:Juno Therapeutics, a Bristol-Myers Squibb Company: Current Employment; Bristol-Myers Squibb: Current equity holder in publicly-traded company. Yang:Juno Therapeutics, a Bristol-Myers Squibb Company: Current Employment; Bristol-Myers Squibb: Current equity holder in publicly-traded company. Ogasawara:Bristol-Myers Squibb: Current Employment; Bristol-Myers Squibb: Current equity holder in publicly-traded company. Thorpe:Bristol-Myers Squibb: Current equity holder in publicly-traded company; Juno Therapeutics, a Bristol-Myers Squibb Company: Current Employment. Siddiqi:Astrazenca: Membership on an entity's Board of Directors or advisory committees; PCYC: Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BeiGene: Other: DMC member; Juno Therapeutics, Pharmacyclics LLC, an AbbVie Company, AstraZeneca, Celgene, Kite Pharma, and BeiGene: Consultancy; Pharmacyclics LLC, an AbbVie Company, Juno Therapeutics, KITE Pharma, AstraZeneca, TG Therapeutics, Celgene, Oncternal, and BeiGene: Research Funding; Pharmacyclics LLC, an AbbVie Company, Seattle Genetics, Janssen, and AstraZeneca: Speakers Bureau; AstraZeneca: Other: Travel/accommodations/expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal